Answer : The actual cell potential of the cell is 0.47 V
Step-by-step explanation:
Reaction quotient (Q) : It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.
The given redox reaction is :
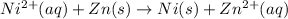
The balanced two-half reactions will be,
Oxidation half reaction :
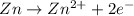
Reduction half reaction :
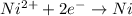
The expression for reaction quotient will be :
![Q=([Zn^(2+)])/([Ni^(2+)])](https://img.qammunity.org/2020/formulas/chemistry/college/qvj5brp12di0t5tx6boets6sat3qecf6xo.png)
In this expression, only gaseous or aqueous states are includes and pure liquid or solid states are omitted.
Now put all the given values in this expression, we get
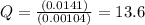
The value of the reaction quotient, Q, for the cell is, 13.6
Now we have to calculate the actual cell potential of the cell.
Using Nernst equation :
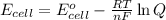
where,
F = Faraday constant = 96500 C
R = gas constant = 8.314 J/mol.K
T = room temperature = 316 K
n = number of electrons in oxidation-reduction reaction = 2 mole
 = standard electrode potential of the cell = 0.51 V
= standard electrode potential of the cell = 0.51 V
 = actual cell potential of the cell = ?
= actual cell potential of the cell = ?
Q = reaction quotient = 13.6
Now put all the given values in the above equation, we get:
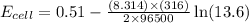
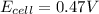
Therefore, the actual cell potential of the cell is 0.47 V