Answer: d. the interpretation of the symbol is "moles of solute per mole of solvent."
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution. It is represented by the symbol M.
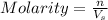
where,
n= moles of solute
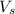 = volume of solution in L
= volume of solution in L
a)
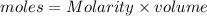
b) According to the neutralization law,

where,
 = molarity of stock solution
= molarity of stock solution
 = volume of stock solution
= volume of stock solution
 = molarity of diluted solution
= molarity of diluted solution
 = volume of diluted solution
= volume of diluted solution
As volume of diluted solution is more, the molarity of diluted solution is less.
c)
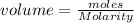
d) The interpretation of the symbol is "moles of solute per Liter of the solution.
Thus the the interpretation of the symbol is "moles of solute per mole of solvent" is incorrect.