Answer: The limiting reagent is barium nitrate, theoretical yield of barium sulfate is 3.03 g and percent yield of barium sulfate is 81.2 %
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
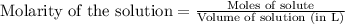 .....(1)
.....(1)
Molarity of potassium sulfate = 1.92 M
Volume of solution = 15.0 mL = 0.015 L (Conversion factor: 1 L = 1000 mL)
Putting values in equation 1, we get:
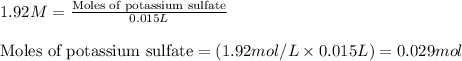
Molarity of barium nitrate = 0.860 M
Volume of solution = 14.9 mL = 0.0149 L
Putting values in equation 1, we get:

For the given chemical reaction:
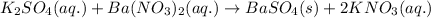
By Stoichiometry of the reaction:
1 mole of barium nitrate reacts with 1 mole of potassium sulfate
So, 0.013 moles of barium nitrate will react with =
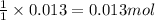 of potassium sulfate.
of potassium sulfate.
As, amount of potassium sulfate is more than the required amount. So, it is considered as an excess reagent.
Thus, barium nitrate is considered as a limiting reagent because it limits the formation of product.
By Stoichiometry of the reaction:
1 mole of barium nitrate produces 1 mole of barium sulfate
So, 0.013 moles of barium nitrate will produce =
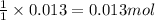 of barium sulfate.
of barium sulfate.
To calculate the mass of barium sulfate, we use the equation:

Molar mass of barium sulfate = 233.4 g/mol
Moles of barium sulfate = 0.013 moles
Putting values in equation 1, we get:

To calculate the percentage yield of barium sulfate, we use the equation:
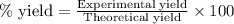
Experimental yield of barium sulfate = 2.46 g
Theoretical yield of barium sulfate = 3.03 g
Putting values in above equation, we get:

Hence, the limiting reagent is barium nitrate, theoretical yield of barium sulfate is 3.03 g and percent yield of barium sulfate is 81.2 %