Answer:
The density of the gas is 1.42g/L and the molar mass is 32.92 g/mol
Step-by-step explanation:
Mass of the unknown gas=m= 4.05g
Temperature=T= 35C
Pressure exerted upon the unknown gas= P= 1 atm
Volume of the flask=V= 2.85 L
Density= ?
Density=
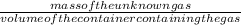
=
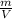
=
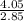
=1.421= 1.42g/L
Molar mass of the gas=M=?
Now,
As the formula is defined
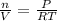
n=
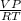
(R= 0.082 L atm /K mol)
Since Temperature= 35C= (35+273) K
=308 K
n=
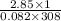
n=
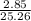
n=0.1129 moles
Molar mass= M=
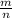
=
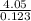
=32.92 g/mol
Hence the density of the gas is 1.42 g/L and the molar mass is 32.92 g/mol