Answer:
B. The volume will triple (increase by a factor of three).
Step-by-step explanation:
From Charles law (Temperature -Volume law):
We know that the volume of a given amount of gas held at constant pressure is directly proportional to the Kelvin temperature.
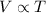
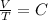 ,c=constant
,c=constant
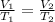
Which means if temperature increases,then volume will also increase.
Here the absolute temperature is increasing by a factor of
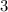 ,so the volume will also increase by a factor of
,so the volume will also increase by a factor of
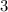 .
.
So our final answer is option B which is that the volume will triple (increase by a factor of 3).