Answer: 0.042
Step-by-step explanation:
The partial pressure of a gas is given by Raoult's law, which is:
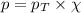
where,
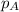 = partial pressure of oxygen = 0.210 atm
= partial pressure of oxygen = 0.210 atm
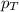 = total pressure = 5.0 atm
= total pressure = 5.0 atm
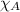 = mole fraction of oxygen = ?
= mole fraction of oxygen = ?
Putting values in above equation, we get:
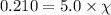
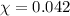
Hence, the mole fraction of oxygen necessary in order for the partial pressure of oxygen in the gas mixture the diver breathes to be 0.210 atm is 0.042.