Answer:
H---H and Cl---Cl bonds are broken and H---Cl bonds are formed.
Step-by-step explanation:
The reaction between Hydrogen and Chlorine to produce HCl is:
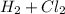 ⇒
⇒

In this reaction the reactants are
 and
and
 .
.
The product is HCl.
The bonds existing in the reactants are
1) H---H bond in
 (Non polar and covalent bond)
(Non polar and covalent bond)
2)Cl---Cl bond in
 ( Non polar and covalent bond)
( Non polar and covalent bond)
The bonds existing in product is/are:
1)H---Cl bond in HCl (Polar and covalent bond)
So all the chemical bonds in the reactants are broken to produce the chemical bonds in the products
In other words,
The H---H bonds and Cl---Cl bonds are broken to produce H---Cl bonds.