Answer:
The value of the turnover number of the enzyme carbonic anhydrase is
 .
.
Step-by-step explanation:
The turnover number is defined as maximum number of conversions of substrate molecules per second by a single catalytic site for given a concentration of enzyme..
Turn over number =
![(R_(max))/([E]_t)](https://img.qammunity.org/2020/formulas/chemistry/college/8e9a9gsra0e26me7rd9m472z800drecdpv.png)
![[E]_t](https://img.qammunity.org/2020/formulas/chemistry/college/ovuq92nyl78dsknddv1b2kj3zs1f6sc9px.png) =catalytic site concentration
=catalytic site concentration
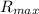 = Maximum reaction rate
= Maximum reaction rate
We are given with:
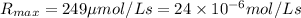
![[E]_t=2.20 nmol/L=2.20* 10^(-9) mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/kzuau9jnd7sfkwubk2fkccqox0f9gzwrj4.png)
Turnover number :
=
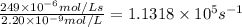
The value of the turnover number of the enzyme carbonic anhydrase is
 .
.