Answer:
The correct answer is option C.
Step-by-step explanation:
Isoelectronic species are those atoms or ions which same number of electrons.But they differ in physical and chemical properties.
Atomic number of nitrogen atom is 7.
![[N]=1s^22s^22p^3](https://img.qammunity.org/2020/formulas/chemistry/college/iopdk4i7tzrvs8r1dtjignq5g4nohb9wjx.png)
![[N]^(3-)=1s^22s^22p^6](https://img.qammunity.org/2020/formulas/chemistry/college/kggpavafzcdg68ipn6gbu6b32l1b2b6tdi.png)
Number electron in nitride ions =
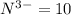
Atomic number of oxygen atom is 8.
![[O]=1s^22s^22p^4](https://img.qammunity.org/2020/formulas/chemistry/high-school/b1p9l8rvjz0s6smf1yzenvexrdu81rit9d.png)
![[O]^(2-)=1s^22s^22p^6](https://img.qammunity.org/2020/formulas/physics/high-school/8chyvjiszxwe215utcebxlcfw0c4csci76.png)
Number electron in oxide ions =
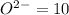
![[Ne]=1s^22s^22p^6](https://img.qammunity.org/2020/formulas/chemistry/college/uztpad118h2ck21sh4xa06uu2kiil4wrmn.png)
Number of electrons neon =
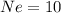
Hence,
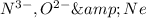 are isoelectronic.
are isoelectronic.