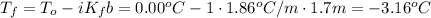Answer:
Step-by-step explanation:
In order to answer this question, we need to be familiar with the law of freezing point depression. The law generally states that mixing our solvent with some particular solute would decrease the freezing point of the solvent.
This may be expressed by the following relationship:
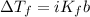
Here:
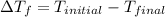 is the change in the freezing point of the solvent given its initial and final freezing point temperature values;
is the change in the freezing point of the solvent given its initial and final freezing point temperature values;
 is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt);
is the van 't Hoff factor (i = 1 for non-electrolyte solutes and i depends on the number of moles of ions released per mole of ionic salt);
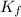 is the freezing point depression constant for the solvent;
is the freezing point depression constant for the solvent;
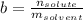 is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).
is molality of the solute, defined as a ratio between the moles of solute and the mass of solvent (in kilograms).
We're assuming that you meant 1.7-molal solution, then:
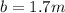
Given ethylene glycol, an organic non-electrolyte solute:
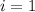
The freezing point depression constant:
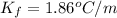
Initial freezing point of pure water:
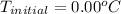
Rearrange the equation for the final freezing point and substitute the variables: