Answer:
The ionization energy for a mole of hydrogen atoms is 1,312.17 kiloJoules.
Step-by-step explanation:
Energy of the nth orbit by Bohr was given by:
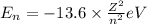
where,
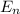 = energy of
= energy of
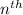 orbit
orbit
n = number of orbit
Z = atomic number
Energy of the first shell(n=1) in hydrogen atom:
Z = 1
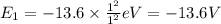
Energy of the first shell(n=∞) in hydrogen atom:
Z = 1
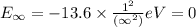
Ionization energy of hydrogen atom,I.E : 1 → ∞

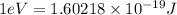
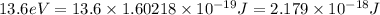
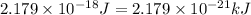 (1kJ = 1000J)
(1kJ = 1000J)
Ionization energy of 1 mol = E
1 mol =
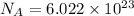 atoms
atoms
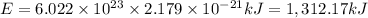
The ionization energy for a mole of hydrogen atoms is 1,312.17 kiloJoules.