Answer:
The magnetic field is 14.08 T.
Step-by-step explanation:
Given that,
Speed of electron
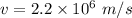
Distance
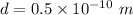
Suppose we need to find the magnetic field at the location of the proton
We need to calculate the magnetic field
Using formula of magnetic field
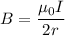 ....(I)
....(I)
Using formula of current
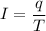
Using formula of time
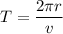
Put the value of current and time in equation (I)
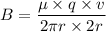
Put the value into the formula
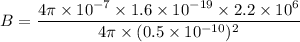
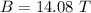
Hence, The magnetic field is 14.08 T.