Answer:
There are necessaries 35,2g of NH₄NO₃ per 100,0g of water to decrease the temperature of the solution from 25,0°C to 5,0°C
Step-by-step explanation:
To decrease the temperature of the solution there are necessaries:
4,184J/g°C×(5,0°C-25,0°C)×(100,0g+X) = -Y
8368J + 83,68J/gX = Y (1)
Where x are grams of NH₄NO₃ you need to add and Y is the energy that you need to decrease the heat.
Also, the energy Y will be:
Y = 25700J/mol×
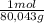 X
X
Y = 321J/g X (2)
Replacing (2) in (1)
8368J + 83,68J/g X = 321J/g X
8363J = 237,32J/gX
X = 35,2g
Thus, there are necessaries 35,2g of NH₄NO₃ per 100,0g of water to decrease the temperature of the solution from 25,0°C to 5,0°C
I hope it helps!