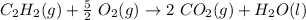Answer:
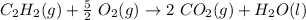
Step-by-step explanation:
Acetylene is an organic compound consisting of only carbon and hydrogen atoms, also known as a pure hydrocarbon.
Every hydrocarbon might undergo either an incomplete or a complete combustion. In an incomplete combustion, carbon monoxide is produced along with water, while in a complete combustion, there's enough oxygen to oxidize the resultant carbon monoxide to carbon dioxide.
In a complete combustion of acetylene, it reacts with oxygen to produce carbon dioxide and water: