To develop this problem it is necessary to collect the concepts related to Ohm's Law.
Ohm's law can be expressed as:
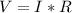
Where
V = Voltage
I = Current
R = Resistance
For our problem the total values of the variables would be given by:
 Two batteries
Two batteries
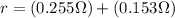
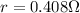
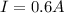
PART A) Whereas the lamp resistance is a surplus greater than the previous resistance given, it must be
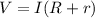
Where R is the resistance of the lamp,
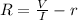
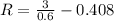
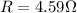
PART B) To calculate the percentage difference in the internal potential we start by calculating the internal voltage given by
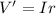
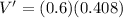
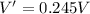
Then the fraction of energy trasnferred is
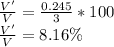
Therefore the fraction of the chemical energy transformed is 8.16%