Answer:
The molarity of KOH used is 0.04 M
Explanation:
Given base used is KOH and the acid used is Acetic Acid
According to titration;
M1V1 = M2V2
Where, M1 is the molarity of acid
V1 is the volume of acid
M2 is the molarity of base
V2 is the volume of base
Given,
Volume of KOH (base) – V2 = 25 mL
Volume of acetic acid (acid) used – V1 = 200 mL
Molarity of acetic acid M1 = 0.0050 M
Substituting the values;
0.0050 × 200 = M2 × 25
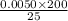
M2 = 0.04 M
The molarity of KOH used is 0.04 M