Answer:
4.51 × 10³ kcal
B. less
Step-by-step explanation:
Let's consider the combustion of 2-methylheptane.
C₈H₁₈ + 12.5 O₂ ⇒ 8 CO₂ + 9 H₂O
When 1 mole of C₈H₁₈ burns, 1.306 × 10³ Kcal are released (heat of combustion). The molar mass of C₈H₁₈ is 114 g/mol. Then, for 394 g of C₈H₁₈:
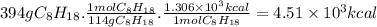
Assuming the same efficiency, would 394 grams of ethanol provide more, less, or the same amount of energy as 394 grams of 2-methylheptane?
Let's consider the combustion of ethanol.
C₂H₆O + 3 O₂ ⇒ 2 CO₂ + 3 H₂O
When 1 mole of C₂H₆O burns, 326.7 Kcal are released (heat of combustion). The molar mass of C₂H₆O is 46.0 g/mol. Then, for 394 g of C₂H₆O:
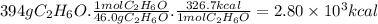
The combustion of 394 g of ethanol provides less energy than the combustion of 394 g of 2-methylheptane.