Answer:
Final energy will be 40 KJ
Step-by-step explanation:
We have given that 30 KJ of the heat is transferred to the water
So
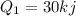
And 5 KJ of heat is lost to the surrounding air
So
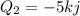
So total heat
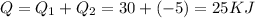
The paddle-wheel work amounts to 5000 N -m
So work done W = -5 KJ ( as work is done by the paddle wheel )
We know that
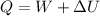
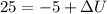
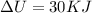
We have given initial kinetic energy = 10 KJ
So
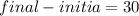
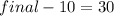
So final energy = 30+10 =40 KJ