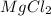Answer:
3.6 moles of
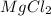 are present in 342 g of
are present in 342 g of
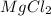
Explanation:
Given the weight of compound
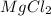 = 342 g
= 342 g
Atomic Mass of Mg = 24 amu
Atomic Mass of Cl = 35.5 amu
Computing the molar mass of
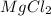 =
=
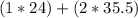 amu = 24 + 71 = 95 amu
amu = 24 + 71 = 95 amu
No. of moles of a compound is calculated by the formula =
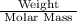
Substituting the value,
Moles =
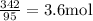
Therefore, 3.6 moles of
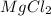 are present in 342 g of
are present in 342 g of