Answer:
0.109 L of
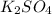 will be needed
will be needed
Step-by-step explanation:
Given the molarity of
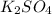 is 3.86M
is 3.86M
Molar mass of
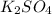 = 174.01 g/mol
= 174.01 g/mol
Weight/Mass of solute
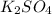 is given as 72.9 g
is given as 72.9 g
Moles of
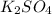 based on the values given
based on the values given
=
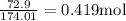
By definition,
Molarity =
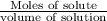
And thus, volume =
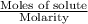
Substituting the values in the above formula,
Volume =
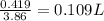
Therefore, 0.109 L of
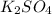 will be needed
will be needed