To develop this problem we require the concepts related to wavelength and its expression to calculate it.
The wavelength is given by
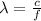
Where,
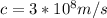 light velocity
light velocity
f = frequency.
Our values are given by,
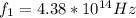
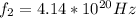
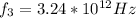
Then,
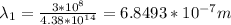 Visible
Visible
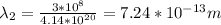 Gamma Ray
Gamma Ray
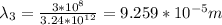 Infrared
Infrared
*Note the designation on the type of rays that are, can be found in consulted via On-line or in the optical books referring to the electromagnetic spectrum table with their respective ranges.