Answer: The chemical equation is written below.
Step-by-step explanation:
Decomposition reaction is defined as the chemical reaction in which a single large substance breaks down into two or more smaller substances.
We are given a chemical compound having formula
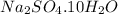
The chemical equation for the decomposition of given hydrated salt follows:
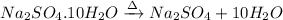
The hydrated salt looses its water of crystallization and forms anhydrous salt.
Hence, the chemical equation is written above.