Answer:
The following statement is true
When 137.14 g PCl₃(l) react, 304.0 kJ are consumed.
Step-by-step explanation:
The given equation is
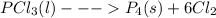
Molecular weight of
PCl₃ = 137.14
P₄ = 123.88
The enthalpy is positive which means the reaction is endothermic.
The reaction will absorb energy from the surroundings.
A) When 1 mol PCl₃(l) reacts, 304.0 kJ are released: False, energy is being absorbed not released
B) When 1 mol P₄(s) is produced, 304.0 kJ are consumed: False as the reverse reaction will be exothermic and there will be release of energy during formation of phosphorus.
C) When 548.56 g PCl₃(l) react, 304.0 kJ are released: Energy is being consumed so it is false
D) When 137.14 g PCl₃(l) react, 304.0 kJ are consumed:True as the enthalpy of reaction is 304.0 kJ
E) When 123.88 g P₄(s) are produced, 304.0 kJ are released: Energy is being consumed so the statement is False.