Answer:
A. Volume is unchanged
Step-by-step explanation:
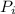 = initial pressure of the gas = 722 torr = 96258.7 pa
= initial pressure of the gas = 722 torr = 96258.7 pa
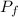 = final pressure of the gas = 0.950 atm = 96258.75 pa
= final pressure of the gas = 0.950 atm = 96258.75 pa
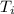 = initial temperature = 32 °F = 272.15 K
= initial temperature = 32 °F = 272.15 K
 = final temperature = 273 K
= final temperature = 273 K
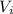 = initial volume
= initial volume
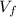 = final volume
= final volume
Using the Equation
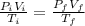
Inserting the values
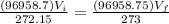
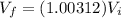
Hence the volume is unchanged.