Answer:
The needed energy to melt of ice is 1670 J.
Step-by-step explanation:
Given that,
Mass of ice = 5 g
Specific latent heat = 334000 J/kg
We need to calculate the energy
Using formula of energy
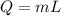
Where, m = mass
L = latent heat
Put the value into the formula
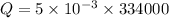
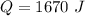
Hence, The needed energy to melt of ice is 1670 J.