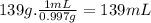Answer:
The volume of water is 139 mL.
Step-by-step explanation:
Due to the Law of conservation of energy, the heat lost by coffee is equal to the heat gained by the water, that is, the sum of heats is equal to zero.

The heat (Q) can be calculated using the following expression:
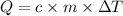
where,
c is the specific heat of each substance
m is the mass of each substance
ΔT is the difference in temperature for each substance
The mass of coffee is:
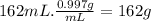
Then,
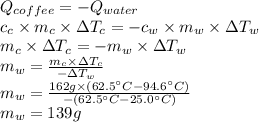
The volume of water is: