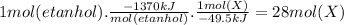Answer:
28 moles of X.
Step-by-step explanation:
Let's consider the complete combustion of ethanol.
C₂H₆O(l) + 3 O₂(g) ⇒ 2 CO₂(g) + 3 H₂O(l)
When 1 mole of ethanol burns it releases 1370 kJ, that is, the enthalpy of combustion is -1370 kJ/mol. If the energy released by one mole of etanol were used to syntesize the compound X, it would take 45.9 kJ to form 1 mole of X, that is, the enthalpy of formation is -49.5 kJ/mol. Then,