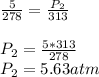Answer:
a) 5.63 atm
Step-by-step explanation:
We can use combined gas law
The combined gas law combines the three gas laws:
- Boyle's Law, (P₁V₁ =P₂V₂)
- Charles' Law (V₁/T₁ =V₂/T₂)
- Gay-Lussac's Law. (P₁/T₁ =P₂/T₂)
It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant.
P₁V₁/T₁ =P₂V₂/T₂
where P = Pressure, T = Absolute temperature, V = Volume occupied
The volume of the system remains constant,
So, P₁/T₁ =P₂/T₂
a)