Answer: The net chemical equation for the production of acrylic acid is given below.
Step-by-step explanation:
We are given two intermediate equations:
Equation 1:
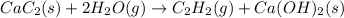
Equation 2:
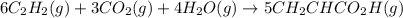
To get the net chemical equation for the formation of acrylic acid from calcium carbide, carbon dioxide and water, we multiply the first equation by a factor of '6'.
The equation becomes:
Equation 1:
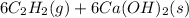
Equation 2:
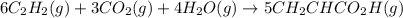
Net chemical equation now becomes:
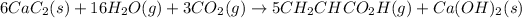
Hence, the net chemical equation for the production of acrylic acid is given above.