Answer:
5.5g of ice melts when a 50g chunk of iron at 80°C is dropped into a cavity
Step-by-step explanation:
The concept to solve this problem is given by Energy Transferred, the equation is given by,
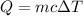
Where,
Q= Energy transferred
m = mass of water
c = specific heat capacity
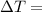 Temperature change (K or °C)
Temperature change (K or °C)
Replacing the values where mass is 50g and temperature is 80°C to 0°C we have,
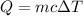
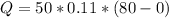
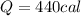
Then we can calculate the heat absorbed by m grams of ice at 0°C, then
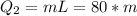
How Q_1=Q_2, so
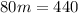
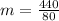
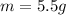
Then 5.5g of ice melts when a 50g chunk of iron at 80°C is dropped into a cavity