Answer:
Rate constant:
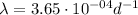
Time to deacy to 1/8 of its original cocentration:
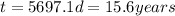
Explanation:
To find the rate constant for the decay process, we use the next equation:
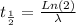
where λ: constant for the decay process, and
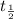 : half-life for the decay
: half-life for the decay
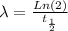
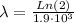
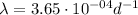
Now, to calculate the time for a sample of 60Co to decay to 1/8th of its original concentration, we use the exponential decay equation:
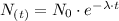
where
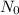 : initial quantity of the 60Co and
: initial quantity of the 60Co and
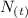 : is the quantity has not yet decayed after a time t.
: is the quantity has not yet decayed after a time t.
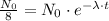
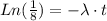
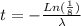
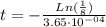
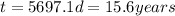
Have a nice day!