Answer:
2.32
Step-by-step explanation:
For the resolution of the problem one of the laws of Charles - Gay Lussac must be used, in which it is stated that if a certain amount of gas is kept at a constant volume (as in this case), its pressure will be directly proportional to its absolute temperature. Mathematically, it can be written as follows:
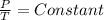 ⇒to constant V
⇒to constant V
In the case of the statement, that we have an initial system (P₁ and T₁) and an final system (P₂ and T₂), the equation can be written:
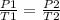
It can be rearranged to clear P₂ which is what needs to be calculated, then:
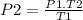
It would only be necessary to convert the temperature units from ° C to K, to have the absolute temperature values. If T (K) = t (° C) + 273.15, then:
T₁ = 0 + 273.15 = 273.15 K
T₂ = 150 + 273.15 = 423.15 K
Now, you can replace the values to the equation and calculate P₂
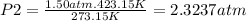 ≅ 2.32 atm
≅ 2.32 atm
As the statement requests the result with three significant figures and no units, the answer is that the final pressure is 2.32