Answer:
b) [bicarbonate]/[carbonic acid] = 9.4
Step-by-step explanation:
The pH of a buffer system is given by the Henderson-Hasselbach equation:
- pH = pKa + log ([bicarbonate] / [carbonic acid])
Where pka = -log (Ka) = -log(4.2x10⁻⁷) = 6.38
Now we solve for [bicarbonate] / [carbonic acid]:
- 7.35 = 6.38 + log ([bicarbonate] / [carbonic acid])
- 0.97 = log ([bicarbonate] / [carbonic acid])
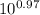 = [bicarbonate] / [carbonic acid]
= [bicarbonate] / [carbonic acid]
- [bicarbonate] / [carbonic acid] = 9.33 ≈ 9.4
So the answer is b).