Answer:
27%
Step-by-step explanation:
Hello,
The following information is missing, but I found it: "1.92 g of sodium sulfate is produced from the reaction of 4.9 g of sulfuric acid and 7.8 g of sodium hydroxide" so the undergoing chemical reaction is:

Now, to compute the percent yield, we must first establish the limiting reagent to subsequently determine the theoretical yield of sodium sulfate because the real (1.92g) is already given, thus, we consider the following procedure:
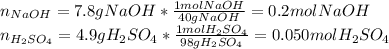
- The moles of sodium hydroxide that completely react with 0.05 moles of sulfuric acid are:
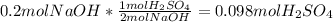
As this number is higher than the previously computed 0.05 moles of available sulfuric acid, one states that the sulfuric acid is the limiting reagent. Now, the theoretical grams of sodium sulfate are found via:
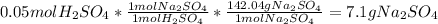
Finally, the percent yield turns out into:
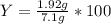
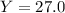 %
%
Best regards.