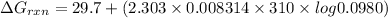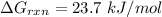Answer:
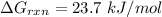
Step-by-step explanation:
According to the reaction,
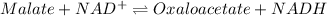
The expression for the equilibrium constant is:-
![K =\frac {[oxaloacetate][NADH]}{[malate][NAD^+]}](https://img.qammunity.org/2020/formulas/chemistry/high-school/yymvg1ouc87wuzulffchsaqobwngyf9okz.png)
Given:-
[malate] = 1.13 mM
[oxaloacetate] = 0.270 mM
![[NAD^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/yhlflqim1yxfi76ni7x50qmvxst27x4yyo.png) = 390 mM
= 390 mM
[NADH] = 160 mM
Thus,
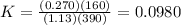
The expression for calculation of free energy change is shown below as:-
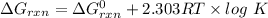
Where, R is gas constant, 8.314 J/K.mol
T is the temperature in Kelvins
So, Given:-
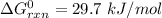
T = 310 K
Thus,