Step-by-step explanation:
1) Reaction between nitrogen gas and oxygen gas.
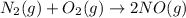
According to reaction, hen 1 mole nitrogen gas reacts with 1 mole of oxygen gas it forms nitrogen oxide gas as a product.
2)reaction between nitrogen oxide and oxygen gas.
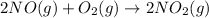
According to reaction , when 2 mole of nitrogen oxide reacts with 1 moles of oxygen gas to form 2 moles of nitrogen dioxide.