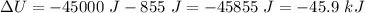Answer:
D. - 45.9 kJ
Step-by-step explanation:
According to the first law of thermodynamics:-
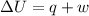
Where,
U is the internal energy
q is the heat
w is the work done
From the question,
q = - 45.0 kJ = - 45000 J ( 1 kJ = 1000 J ) (+ sign as the heat is being released)
w = 855 J (work is done on the surroundings)
So, work done by the system = - 855 J
So,