Answer:
(a)
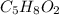
(b)
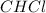
(c)
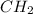
(d)
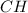
(e)
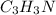
Step-by-step explanation:
Hello,
(a) For the lucite, one computes the moles of C, H and O that are present:
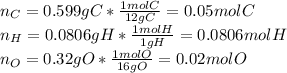
Now, dividing each moles by the smallest moles (oxygen's moles), one obtains:
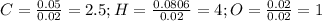
Finally, we look for the smallest whole number subscript by multiplying by 2, so the empirical formula turns out into:
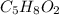
(b) For the Saran, one computes the moles of C, H and Cl that are present:
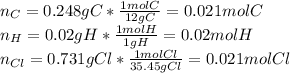
Now, dividing each moles by the smallest moles (hydrogen's moles), one obtains:
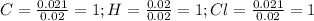
Finally, as all of the subscripts are whole numbers, the empirical formula turns out into:
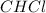
(c) For the polyethylene, one computes the moles of C and H that are present:
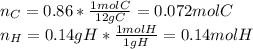
Now, dividing each moles by the smallest moles (carbon's moles), one obtains:
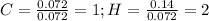
Finally, as all of the subscripts are whole numbers, the empirical formula turns out into:
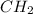
(d) For the polystyrene, one computes the moles of C and H that are present:
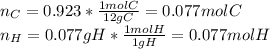
Now, dividing each moles by the smallest moles (either carbon's or hydrogen's moles), one obtains:
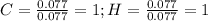
Finally, as all of the subscripts are whole numbers, the empirical formula turns out into:
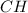
(e) For the orlon, one computes the moles of C, H and N that are present:
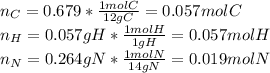
Now, dividing each moles by the smallest moles (nitrogen's moles), one obtains:
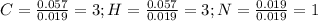
Finally, as all of the subscripts are whole numbers, the empirical formula turns out into:
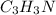
Best regards.