Answer:
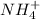 is stronger acid than HCN
is stronger acid than HCN
Step-by-step explanation:
For a acid-conjugate base or base-conjugate acid pair-
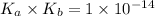
Where,
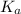 is acid dissociation constant of acid or conjugate acid and
is acid dissociation constant of acid or conjugate acid and
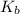 is base dissociation constant of base or conjugate base
is base dissociation constant of base or conjugate base
So,
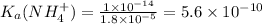
As higher the
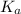 value of an acid , higher will be the dissociation of the acid and hence more stronger will be the acid.
value of an acid , higher will be the dissociation of the acid and hence more stronger will be the acid.
As
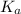 value of
value of
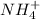 is higher than
is higher than
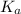 value of HCN therefore
value of HCN therefore
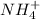 is stronger acid than HCN
is stronger acid than HCN