4.648 gm of solute is needed to make 37.5 mL of 0.750 M KI solution.
Solution:
We will start with the Molarity
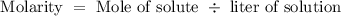
Also we know 1000 ml = 1 L
Therefore 37.5 ml by 1000ml we obtained 0.0375L
Equation for solving mole of solute
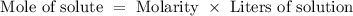
Now, multiply 0.750M by 0.0375
Substitute the known values in the above equation we get
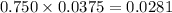
Also we know that Molar mass of KI is 166 g/mol
So divide the molar mass value to get the no of grams.
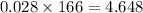
So 4.648 gm of Solute is required for make 37.5 mL of 0.750 M KI solution.