Answer:
he total heat capacity of the calorimeter is - 0.09 kJ/K
Step-by-step explanation:
Given information
the combustion energy of glucose E = 15.57 kJ/g
mass of glucose, m = 1.500 g
initial temperature,
 = 21.45
= 21.45
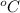
final temperature
 = 23.34
= 23.34
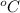
the heat capacity of the calorimeter can be determined by
C = Q/ΔT
where
C = heat capacity (kJ/
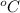 )
)
Q = quantity of the heat (kJ)
ΔT = temeperature difference (
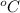 )
)
Q = E m
= - (15.57 kJ/g)(1.500 g)
= 23.355 kJ
ΔT =
 -
-

= 23.34
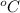 - 21.45
- 21.45
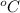
= 1.89
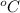
Since the question ask to answer in kJ/k, we need to convert the temperature from celcius to kelvin by adding 273. thus,
ΔT = 1.89
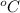 + 273
+ 273
= 274.89 K
C = Q/ΔT
= - 23.355 kJ/274.89 K
= - 0.09 kJ/K