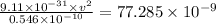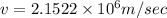Answer:
(a) Electric force will be
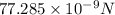
(B) Velocity
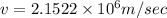
Step-by-step explanation:
We have given that radius of the circular orbit
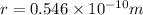
Charge on electron

(A) According to coulomb's law electric force between two charges is given by
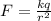
So electric force
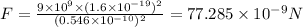
We know that centripetal force is given by
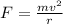
So