Answer :
(a) The value of
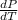 is
is
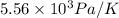
(b) The percentage error will be 2.5 %
Explanation :
(a) First we have to calculate the
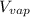 .
.
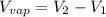
 = volume of liquid =
= volume of liquid =
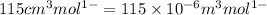
 = volume of vapor =
= volume of vapor =
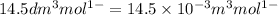
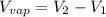
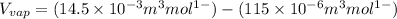
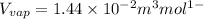
Now we have to calculate the value of
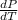
The Clausius- Clapeyron equation is :
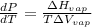
where,
T = temperature = 180 K
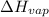 = heat of vaporization = 14.4 kJ/mole = 14400 J/mole
= heat of vaporization = 14.4 kJ/mole = 14400 J/mole
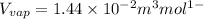
Now put all the given values in the above formula, we get:
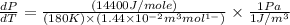
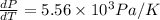
(b) Now we have to calculate the percentage error.
Now we have to calculate the value of
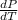 at normal boiling point.
at normal boiling point.
The Clausius- Clapeyron equation is :
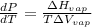
As we know that : PV = nRT
So,
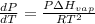
where,
R = gas constant = 8.314 J/K.mol
T = temperature = 180 K
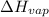 = heat of vaporization = 14.4 kJ/mole = 14400 J/mole
= heat of vaporization = 14.4 kJ/mole = 14400 J/mole
P = pressure at normal boiling point = 101325 Pa
Now put all the given values in the above formula, we get:
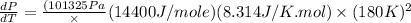
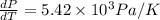
Now we have to determine percentage error.
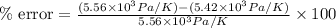
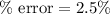
Therefore, the percentage error will be 2.5 %