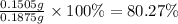Answer:
80.27%
Step-by-step explanation:
Let's consider the following balanced equation.
2 Fe³⁺(aq) + Sn²⁺(aq) ⇒ 2Fe²⁺(aq) + Sn⁴⁺(aq)
First, we have to calculate the moles of Sn²⁺ that react.
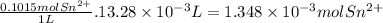
We also know the following relations:
- According to the balanced equation, 1 mole of Sn²⁺ reacts with 2 moles of Fe³⁺.
- 1 mole of Fe³⁺ is oxidized from 1 mole of Fe.
- The molar mass of Fe is 55.84 g/mol.
Then, for 1.348 × 10⁻3 moles of Sn²⁺:
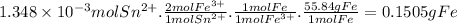
If there are 0.1505 g of Fe in a 0.1875 g sample, the mass percentage of Fe is: