Answer:
The number of half-lives that have occurred are 4.
Step-by-step explanation:
Formula used for number of half lives :
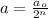
where,
a = amount of reactant left after n-half lives
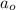 = Initial amount of the reactant
= Initial amount of the reactant
n = number of half lives
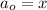
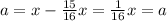
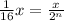
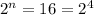
n = 4
The number of half-lives that have occurred are 4.