Answer:

Step-by-step explanation
Density can be found by dividing the mass by the volume.
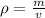
We know the iron sheet has a mass of 28.5 grams and the volume is 3.60 milliliters.
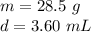
Substitute the values into the formula.
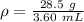
Divide.
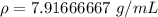
Let's round to the nearest thousandth.
The 6 in the ten-thousandth tells us to round the 6 to a 7.
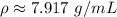
The density of iron is about 7.917 grams per milliliter.