Answer:
The molarity of the HCl solution is 0.17M
Step-by-step explanation:
When a titration occurs between an acid and a base it is called neutralization.
The formula to calculate the concentration of the acid is:
Ca*Va=Cb*Vb
where Ca and Cb are the concentrations of the acid and the base respectively and Va and Vb are the volumes of the acid and the base.
As HCl is an acid, solve the equation for Ca:
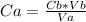
Replacing values:
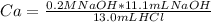
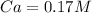
Therefore the molarity of the HCl solution is 0.17M