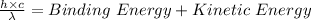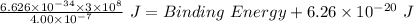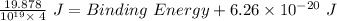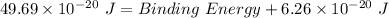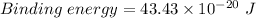Answer:
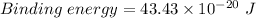
Step-by-step explanation:
Using the expression for the photoelectric effect as:
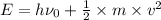
Also,
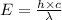
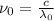
Applying the equation as:
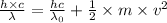
Where,
h is Plank's constant having value
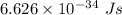
c is the speed of light having value
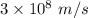
 is the wavelength of the light being bombarded
is the wavelength of the light being bombarded
Given,
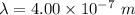
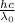 is the binding energy or threshold energy
is the binding energy or threshold energy
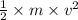 is the kinetic energy of the electron emitted. =
is the kinetic energy of the electron emitted. =
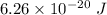
Thus, applying values as: