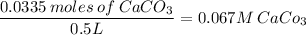Answer:
a. 8.75 M NaOH
b. 0.425 M CuCl₂ or 0.43 M CuCl₂
c. 0.067 M CaCO₃ or 0.07 M CaCO₃
Explanation:
Molality is computed using the formula:
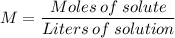
So first thing you need to do is determine how many moles of solute there are and divide it by the solution in liters.
Converting mass to moles, you need to get the mass of each solute per mole. You can use the periodic table to get the atomic mass (which is the grams per mole of each atom) of each of the elements involved. Then add them up and you will have how many grams per mole of each compound.
1. 35.0g of NaOH in 100ml H₂O
Element number of atoms atomic mass TOTAL
Na 1 x 22.99g/mol = 22.99g/mol
O 1 x 16.00g/mol = 16.00g/mol
H 1 x 1.01g/mol = 1.01g/mol
40.00g/mol
This means that the molecular mass of NaOH is 40.00 g/mol
Then we use this to convert 35.0g of NaOH to moles:
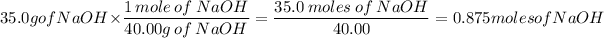
Now that you have the number of moles we divide it by the solution in liters. Before we can do that you have to conver 100ml to L.
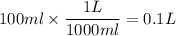
Then we divide it:
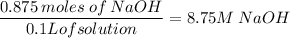
2. 20.0g CuCl₂ in 350ml H₂O
Element number of atoms atomic mass TOTAL
Cu 1 x 63.55g/mol = 63.55g/mol
Cl 2 x 34.45g/mol = 70.90g/mol
134.45g/mol
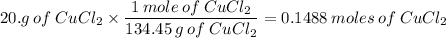
350ml = 0.350L
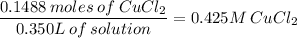
3. 3.35g CaCO₃ in 500ml
Element number of atoms atomic mass TOTAL
Ca 1 x 40.08g/mol = 40.08g/mol
C 1 x 12.01g/mol = 12.01g/mol
O 3 x 16.00g/mol = 48.00g/mol
100.09g/mol
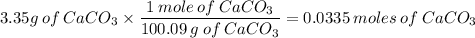
500ml = 0.5L