Answer : The given image is shown below.
Explanation :
An alkali metal : These are the elements which lie in group 1.
Their general electronic configuration is:
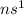 where n is the outermost shell.
where n is the outermost shell.
An alkaline earth metal : These are the elements which lie in group 2.
Their general electronic configuration is:
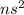 where n is the outermost shell.
where n is the outermost shell.
Metalloids : These are the elements which shows both the property of metals and non-metals that means the metalloids are present in between the metals and non- metals elements.
Noble gas : These are the gases which lie in group 18.
Their general electronic configuration is:
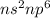 where n is the outermost shell.
where n is the outermost shell.
Halogen : These are the elements which lie in group 17.
Their general electronic configuration is:
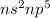 where n is the outermost shell.
where n is the outermost shell.
Transition elements : They are the elements which lie between 's' and 'p' block elements. These are the elements which lie in group 3 to 12. The valence electrons of these elements enter d-orbital.
Their general electronic configuration is:
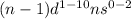 where n is the outermost shell.
where n is the outermost shell.
Lanthanoids : These are the elements in which the last electrons enters one of 4f-orbitals are called lanthanoids.
Actinoids : These are the elements in which the last electrons enters one of 5f-orbitals are called lanthanoids.
In the periodic table, the metals are present on the left side, non-metals are present on the right side and the metalloids are present between the metals and non-metals.